Definite diagnosis of Alzheimer's disease and many neurodegenerative diseases is still at autopsy. In vivo tau PET imaging is one of those game changers in dementia - being able to visualize tau deposition in living individuals for different pathologies will allow discrimination between neurodegenerative diseases, including different tauopathies, and monitoring of disease progression. The challenge though has been in the variety and complexity of development of tau PET tracers that would respond to the different types of tau deposits in the different neurodegenerative diseases.
A team of investigators sought to answer this question: How to fully characterize the binding properties of the tau PET tracers, and to assess their usefulness as an early biomarker of the underlying pathology?

Lea Grinberg, MD, PhD and Duygu Tosun-Turgut, PhD are UCSF Center for Intelligent Imaging (ci2) members who were authors on this work together with Daniela Ushizima, PhD, at Lawrence Berkeley National Laboratory, Berkeley. Dr. Grinberg is the principal investigator of the Grinberg Lab, and the primary aim of Dr. Tosun's research, as the director of the Center for Medical Imaging Informatics and Artificial Intelligence, is multi-disciplinary and multi-modality biomarker discovery for detecting the changes associated with disease specific neuropathology, improving understanding of pathophysiological progression and potentially providing a means of monitoring the efficacy and regional specificity of drug therapy for neurodegenerative diseases.
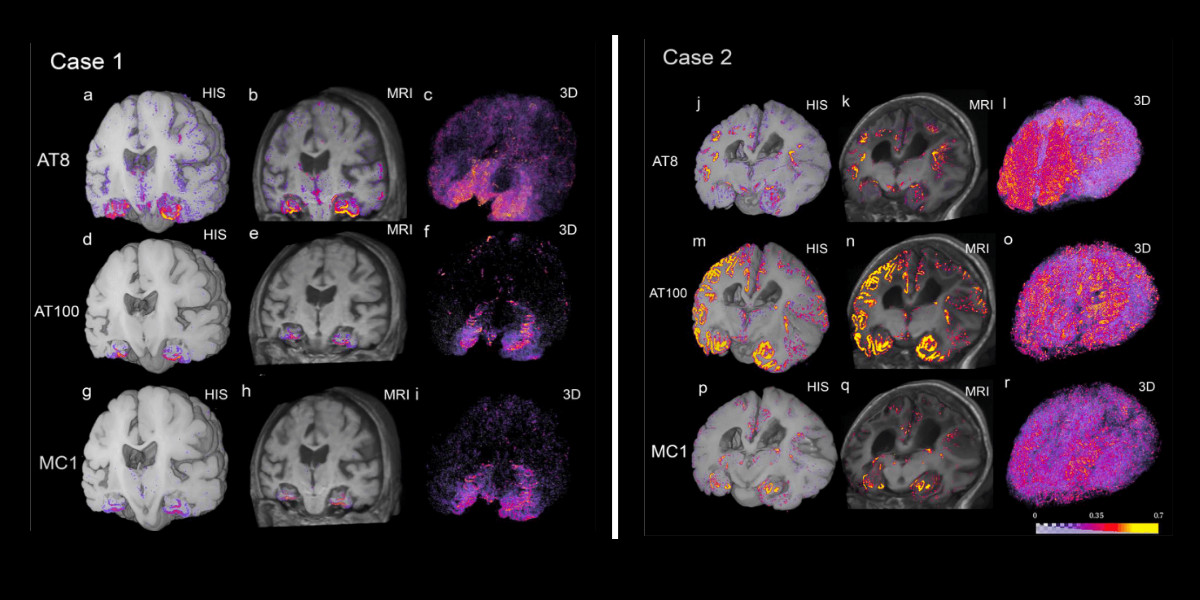
"The overarching goal is to assess the usefulness of tau PET as an early biomarker of the underlying pathology, but we first need to fully characterize the binding properties of the tau PET tracers before answering that question," explains Dr. Tosun. "This work outlines an end-to-end deep learning-powered pipeline to facilitate dense quantification of whole-brain tau inclusions observed at autopsy in perfect alignment with in vivo neuroimaging, which will allow directly linking imaging measures to ground-truth neuropathological inclusions at autopsy."
Their paper was recently published online in NeuroImage. "This work is the result of over 15 years of development from a large multi-institutional team and it shows the power of teamwork in solving complex problems in neuroscience. I always dreamed of being able to see in living patients what I can see under the scope in postmortem tissue. This pipeline makes this dream comes true," said Dr. Grinberg.

Dr. Grinberg's lab is part of the UCSF Memory and Aging Center. Their unique approach allows for simultaneous investigation of several aspects of the complex pathogenesis of neurodegenerative disease putting us in a strategic position for identifying therapeutic targets relevant to the earliest stages of these diseases years before the first clinical manifestations. Current and former lab members that were part of this work include Jonathan Chen, Dulce Ovando, Maryana Alegro, Rana Eser, Wing Hung Lee, Anubhav Shankar, Namrata Kantamneni, Kinson Poon and Shruti Satrawada
Additional authors include Edson Amaro, Jr. and Helmut Heinsen, University of Sao Paulo Medical School (Brazil).



